
- About Us
- Medical School
- Graduate School
- Research
- Research TOP
- Research Departments
- Research Topics
- Research Facilities
- Intellectual Property Promotion Center(TLO)
- Affiliated Hospitals
Department of Biochemistry and Molecular Biology
Research outline (Oishi Lab)
Cardiovascular disease, as a consequence of the obesity related metabolic syndrome, remains a significant cause of morbidity and mortality in industrialized societies. A major effort of our laboratory has been to investigate the molecular mechanism of metabolic syndrome from the viewpoint of transcriptional/epigenetic regulation.
In particular, we focus on skeletal muscle as one of the major metabolic organs. Skeletal muscle consume ~40% of total energy, playing a key role for the pathogenesis of metabolic syndrome. Sarcopenia is defined as a degenerative loss of skeletal muscle mass, quality and strength associated with aging, and obese individuals accompanied with sarcopnia has the highest risk of developing metabolic syndrome.
The long-term goals of our current study are to elucidate: 1) elucidate the mechanism of chronic inflammation leads to metabolic syndrome and 2) the mechanism responsible for pathogenesis of sarcopenia and skeletal muscle degeneration.
1.Mechanisms of chronic inflammation
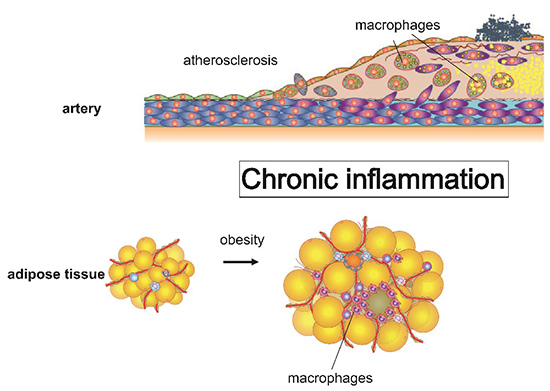
Chronic inflammation is attracting attention as a common underlying condition to obesity, metabolic diseases, neurodegenerative diseases and even cancer over a decade. Chronic inflammation can be thought of as a condition in which inflammation that begins as a response to various stresses does not converge properly. However, the mechanism of why inflammation becomes chronic is not clear.
Recent study suggested that macrophages are diverse cell population, respond to various stimuli to play important role in developing chronic inflammation. Therefore, we focused on macrophages, investigate the mechanism that causes chronic inflammation by using molecular biological methods and the latest genome-wide transcriptome / epigenetic analysis including single-cell transcriptome analysis. In particular, we are also paying attention to the coordinated regulation between cell function and cellar metabolism. By conducting these studies, we aim to develop new effective preventive and therapeutic methods for metabolic diseases such as obesity and atherosclerosis.
2.Mechanisms of age-related regenerative failure and sarcopenia
Skeletal muscle plays an important role not only in exercise and maintaining posture, but also in energy metabolism as the largest glucose-metabolizing organ in the body. Muscle atrophy due to lack of exercise, bedrest, or aging not only causes a decline in motor function, but also increases the risk of metabolic syndrome, which has a great impact on the quality of life.
Skeletal muscle is an organ with high regenerative ability. Satellite cells, muscle-specific stem cells, play an important role in regeneration after muscle damage. When muscle damage occurs, satellite cells become active, proliferate and differentiate into new muscle fibers. In addition, muscle satellite cells replicate themselves and maintain a constant number at all times to prepare for the next injury and contribute to maintaining the homeostasis. It is also known that, many immune cells including macrophages are infiltrated after muscle injury. It is expected that macrophages communicate with satellite cell to regulate regeneration process, however, its molecular mechanism is not clarified.
In our laboratory, we conduct experiments to elucidate the interaction between muscle satellite cells and immune cells in skeletal muscle, and understand the mechanism by which regeneration and inflammation are coordinated and controlled in damaged skeletal muscle. We aim to develop the preventive method and possible treatment for sarcopenia.
Research outline (Toshio Iwasaki, Ph.D.)
The structure of a metal site in metalloenzymes critically influences the fine-tuning of some of the most complicated reactions in the chemistry of life processes. Iron-sulfur (Fe-S) cluster prosthetic groups, consisting of nonheme iron and acid-labile inorganic sulfide atoms, are functionally highly versatile and may be among the most ancient modular metallo-cofactors that might have sustained many indispensable chemical processes in the early evolution of life (such as the hydrogen, nitrogen, carbon and sulfur metabolisms, and anaerobic respiration). All major electron transfer chains in biology incorporate Fe-S clusters, and their roles in catalysis of energy conversion are of central importance in aerobic respiration, photosynthesis, hydrogen metabolism, and a number of interrelated metabolic processes that drive the biosphere on Earth. In addition to these redox and catalytic roles, Fe-S proteins are also known to participate in environmental sensing and gene regulation, and more recently are suggested to be potentially involved in several human diseases (e.g., Parkinson's disease, Friedreich's ataxia). Thus, the potential relationship between some metalloenzymes and human healthcare is also at an interesting stage of development, where a main focus is on the structure-function-physiology interface.
Major determinants of the redox and/or catalytic reactivity of Fe-S enzymes critically depend on the immediate protein environment and its interplay with the electronic structure of the cluster, which can be best addressed through the combined efforts of spectroscopy and protein crystallography: the lack of information thereof translates to uncertainties in the theoretical analysis. Our understanding of these determinants helps not only to explain observations but also to generate systems of predictable function.
* Follow this link to learn more:

